Gold Mass Number
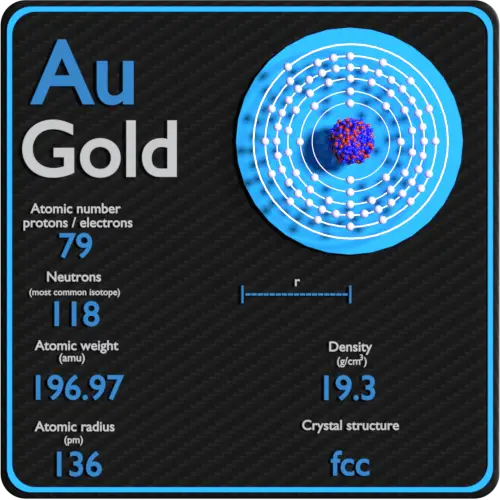
Gold, (symbol Au) has an atomic number of 79 i.e. Each gold atom has 79 protons in its nucleus. The atomic mass of the gold atom is 196.967 and the atomic radius is 0.1442nm. Interestingly this is smaller than would be predicted by theory. The arrangement of outer electrons around the gold nucleus is related to gold's characteristic yellow color. Element Gold (Au), Group 11, Atomic Number 79, d-block, Mass 196.967. Sources, facts, uses, scarcity (SRI), podcasts, alchemical symbols, videos and images.
Gold Mass Number Rounded
What are the numbers of the subatomic particles in gold?
1 Answer
A Gold (Au) atom has 79 protons and 79 electrons. A typical gold atom has 118 neutrons, though there are 18 other radioisotopes discovered so far.
From the periodic table, a gold atom is represented by
79 is its charge (atomic number), which is both its proton number and electron number.
Microsoft word 2016 for mac. 197 is its mass (atomic mass), which is the sum of its proton number and neutron number. The neutron number can be calculated by subtracting the proton mass from the total mass.
Gold Atomic Mass Number
The mass of the electrons does not contribute significantly to the total mass of the atom and therefore can be ignored.
Related questions
