Boron Isotopes
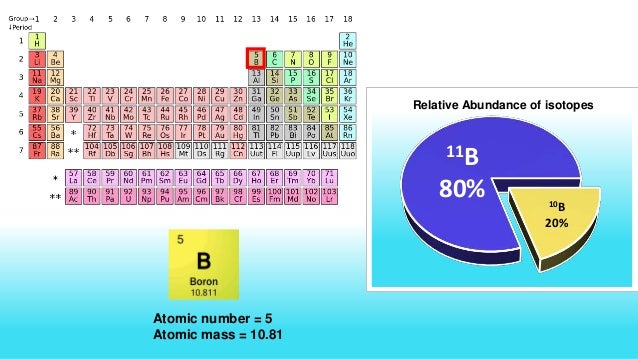
Natural boron consists primarily of two stable isotopes, 11 B (80.1%) and 10B (19.9%). In nuclear industry boron is commonly used as a neutron absorber due to the high neutron cross-section of isotope 10B. Its (n,alpha) reaction cross-section for thermal neutrons is about 3840 barns (for 0.025 eV neutron). The isotopic composition of boron (δ11B) contained in the carbonate shells of marine organisms varies according to pH, from which ocean pH can be reconstructed4,5,6,7,8,9,10,11. Both isotopes of Boron, B-10 and B-11, are used extensively in the nuclear industry. B-10 is used in the form of boric acid as a chemical shim in pressurized water reactors while in the form of sodium pentaborate it is used for standby liquid control systems in boiling water reactors. B-11 can be used as a neutron reflector. Boron has two naturally occurring isotopes 10B and 11B that are stable. 13 radioisotopes of boron are also present with their own half-life. Overview of Isotopes Of Boron Boron is a metalloid in group thirteen with two naturally occurring isotopes 10B and 11B stable in nature having abundances of 0.199(7) and 0.801(7) with the ratio of 4:1.
Boron Isotopes-Based Materials
#G-1110
Development of a Package of Technologies for Commercial Production of Boron Isotopes-Based Materials and Items
Tech Area / Field
Boron Isotopes Numbers
- FIR-ISO/Isotopes/Fission Reactors
- FIR-MAT/Materials/Fission Reactors
Status
8 Project completed
Registration date
10.12.2003
Completion date
30.08.2007
Senior Project Manager
Zalouzhny A A
Leading Institute
National High Technology Center of Georgia, Georgia, Tbilisi
Collaborators
- Spectra Gases, USA, NJ, Branchburg
Project summary
 The objective of the Project is to develop a package of technologies for commercial production of materials and items, based on boron isotopes. The package of technologies will be developed for production facilities comprising 5 separate process sections, working in a single system and providing production of a wide range of boron isotope based materials and items, which are on demand at the world market (10BF3, 11BF3, H310BO3, H311BO3, 10B4C, Fe10B).
The objective of the Project is to develop a package of technologies for commercial production of materials and items, based on boron isotopes. The package of technologies will be developed for production facilities comprising 5 separate process sections, working in a single system and providing production of a wide range of boron isotope based materials and items, which are on demand at the world market (10BF3, 11BF3, H310BO3, H311BO3, 10B4C, Fe10B).Natural boron contains two stable isotopes – boron-10 and boron-11. Their natural abundance in compounds makes approximately 19.3% at. and 80.79% at., respectively. Nuclear properties of boron-10 and boron-11 differ greatly. Their reactions with neutrons are of critical importance. Thermal neutron capture cross sections of 10B and 11B make 4010 Barn and < 0.05 Barn, respectively, and differ more than 80 thousand-fold. These features together with other physical and chemical features make materials, based on the above isotopes widely applicable in various fields of science and nuclear technology.
Application of materials, enriched in 10B: Boron Carbide Control Rods, ZrB2 and HfB2 Neutron Absorbers, Aluminum Boron Alloy Spent Fuel Casks, Superconductivity Mechanism Determination in MgB2 (Isotope Effect), Radiation Hardening (Shielding), Boric Acid Chemical Shim.
Application of 11B enrichedmaterials: Neutron Diffraction Structural Studies, Controlled Doping by Ion implantation (bit flop), Single Event Transient, Neutron induced upsets, Radiation Hardening in Silicon Circuits.
Extension in the application fields and ranges of isotopic materials, based on boron isotopes is limited today, basically due to comparatively high production cost and insufficient scales of production of isotopic raw materials, and drawbacks of the processes of their production. In contrast to the processes of non-isotopic materials production material study of isotopic materials often requires alternative methods and processes, together with general requirements characterized by the highest product yield at all the stages, i.e. the lowest possible losses of expensive isotopic raw material. These circumstances also present specific requirements to the equipment for the isotopic materials production.
Study of the current state of the boric materials market, comparison and generalization of all the above and other important factors provide opportunity to predict substantial growth of demand in isotopic boron containing materials, provided their prices are decreased, and necessary quality and scales of shipment are guaranteed.
These requirements can be met best by the development of a wide-scale production, comprising sections for the production of boron-10 and boron-11 isotopes, sections for reprocessing, purification, testing and certifying desired isotopic materials and items. Economic, energy and isotope saving technologies should be used at all the stages of production.
The proposed Project comprises a set of tasks, the solution of which will allow developing a single package of technologies for wide-scale production of boron isotopes, nuclear and electronic grade materials and items, made on their base.
The works on the Project implementation will be conducted at National High Technology Centre of Georgia (NHTC, Tbilisi), which has been the leading enterprise in the post-soviet area, dealing with separation of isotopes of light elements, such as boron, nitrogen, carbon, oxygen and development and production of labeled compounds and materials on their base.
NHTC has accumulated many-years-long experience in boron isotopes separation and obtaining boron-containing materials and items, their testing and certification.
Development of a package of technologies for commercial production of materials based on boron isotopes is the basic expected result of the Project implementation.
The production complex, which will be designed on the base of the offered package of technologies will consist of 5 separate production sections, working as a single system and providing production of a wide range of boron isotope-based materials and items, which are on demand at the world market: section of isotopes separation; section of the separation process wastes neutralization and utilization; section of boric acid production; section of boron carbide production; section of ferroboron master alloy production. The Department of Analytical Control and Certification will provide services for the above 5 sections.
The proposed Project contains all the stages of research and development works and will be completed by drawing up a whole package of technical documentation, related to the package of technologies; production, testing and certification of experimental lots of materials and items.
Highly efficient technologies for wide-scale and competitive production of Boron-10, Boron-11 isotopes, boron-containing materials and items with preset chemical and isotopic properties will be developed in the result of the Project implementation. In their physical and chemical properties all the obtained materials must meet the requirements of world standards, their specifications will be comparable with those of the leading industrial countries, and besides, in future these materials will find application in atomic energy and engineering, microelectronics and other fields of industry. Technical and economic calculations will be conducted, and a Business-plan on the project results practical application will be drawn-up by the end of the Project implementation.
After the Project completion the results may be used at the interested enterprises with the support of potential investors. NHTC seems to be a probable place of such introduction, as its available production facilities can be extended and improved. A joint-venture with a foreign company may be a possible form of introducing the Project results into practice.
The Project provides opportunity to realize the NHTC objectives, as it provides an opportunity for the former weapon specialists (there are more than 60% of them among the planned Project participants) to reorient their skills and abilities towards peaceful activities; promotes integration of researchers and specialists into international scientific community, promotes researches and developments in the field of environment protection to substantially improve ecological situation and nuclear safety.
Works within the scopes of the Project foresee solution of six basic tasks, each of them being a separate development work.
Task 1. Development of technology for obtaining of Boron-10 and Boron-11 isotopes by the method of chemical isotopic exchange in ВF3 anisole*BF3 system.
Task 2.Development of the processes and technologies providing ecological security of boron isotope production.
Task 3. Development of technologies for the production of boric acid, enriched in Boron-10 and Boron-11 isotopes.
Task 4. Development of technologies for the production of special powders and items from boron carbide, enriched in Boron-10 isotope.
Task 5. Development of technology for the production of ferroboron master-alloy, enriched in Boron-10 isotope.
Task 6. Development of the system of production control, test and certification of boron-containing materials and items, enriched in Boron-10 and Boron-11 isotopes.
By the Project completion (quarters 8 - 9) works, related to technical and economic calculations of the parameters of the package of technologies will be conducted; a Business Plan on the practical application of the Project results will be drawn up (Task 7).

Back
The International Science and Technology Center (ISTC) is an intergovernmental organization connecting scientists from Kazakhstan, Armenia, Tajikistan, Kyrgyzstan, and Georgia with their peers and research organizations in the EU, Japan, Republic of Korea, Norway and the United States.
ISTC facilitates international science projects and assists the global scientific and business community to source and engage with CIS and Georgian institutes that develop or possess an excellence of scientific know-how.
Annual Reports
Annual Reports
The electronic version of the 2019 Annual Report
2019 Audited Financial Report
view
Promotional Material
Значимы проект
See ISTC's new Promotional video view
Subscribe to our newsletters
|
Boron Isotopes Model
U.S. Geological Survey Scientific Investigations Report 2007–5166
By Paul M. Buszka, John Fitzpatrick, Lee R. Watson, and Robert T. Kay
ONLINE ONLY
This report is available below as a 46-page PDF for viewing and printing.
Abstract

Concentrations of boron greater than the U.S. Environmental Protection Agency (USEPA) 900 µg/L removal action level (RAL) standard were detected in water sampled by the USEPA in 2004 from three domestic wells near Beverly Shores, Indiana. The RAL regulates only human-affected concentrations of a constituent. A lack of well logs and screened depth information precluded identification of whether water from sampled wells, and their boron sources, were from human-affected or natural sources in the surficial aquifer, or associated with a previously defined natural, confined aquifer source of boron from the subtill or basal sand aquifers. A geochemically-based classification of the source of boron in ground water could potentially determine the similarity of boron to known sources or mixtures between known sources, or classify whether the relative age of the ground water predated the potential sources of contamination. The U.S. Geological Survey (USGS), in cooperation with the USEPA, investigated the use of a geochemical method that applied boron stable isotopes, and concentrations of boron, tritium, and other constituents to distinguish between natural and human-affected sources of boron in ground water and thereby determine if the RAL was applicable to the situation.
Boron stable-isotope ratios and concentrations of boron in 17 ground-water samples and tritium concentrations in 9 ground-water samples collected in 2004 were used to identify geochemical differences between potential sources of boron in ground water near Beverly Shores, Indiana. Boron and d11B analyses for this investigation were made on unacidified samples to assure consistency of the result with unacidified analyses of d11B values from other investigations. Potential sources of boron included surficial-aquifer water affected by coal-combustion products (CCP) or domestic-wastewater, upward discharge of ground water from confined aquifers, and unaffected water from the surficial aquifer that was distant from human-affected boron sources.
Boron concentrations in potential ground-water sources of boron were largest (15,700 to 24,400 µg/L) in samples of CCP-affected surficial aquifer water from four wells at a CCP landfill and smallest (27 to 63 µg/L) in three wells in the surficial aquifer that were distant from human-affected boron sources. Boron concentrations in water from the basal sand aquifer ranged from 656 µg/L to 1,800 µg/L. Boron concentrations in water from three domestic-wastewater-affected surficial aquifer wells ranged from 84 to 387 µg/L. Among the representative ground-water samples, boron concentrations from all four samples of CCP-affected surficial aquifer water and four of five samples of water from the basal sand aquifer had concentrations greater than the RAL. A comparison of boron concentrations in acid-preserved and unacidified samples indicated that boron concentrations reported for this investigation may be from about 11 to 16 percent less than would be reported in a standard analysis of an acidified sample.
The stable isotope boron-11 was most enriched in comparison to boron-10 in ground water from a confined aquifer, the basal sand aquifer (d11B, 24.6 to 34.0 per mil, five samples); it was most depleted in CCP-affected water from the surficial aquifer (d11B, 0.1 to 6.6 per mil, four samples). Domestic-wastewater-affected water from the surficial aquifer (d11B, 8.7 to 11.7 per mil, four samples) was enriched in boron-11, in comparison to individual samples of a borax detergent additive and a detergent with perborate bleach; it was intermediate in composition between basal sand aquifer water and CCP-affected water from the surficial aquifer. The similarity between a ground-water sample from the surficial aquifer and a hypothetical mixture of unaffected surficial aquifer and basal sand aquifer waters indicates the potential for long-term upward discharge of ground water into the surficial aquifer from one or more confined aquifers. Estimated d11B values for acidified samples were depleted by 1.9 to 2.8 per mil in comparison to unacidified samples from the four wells sampled; those differences were small in comparison to the differences between d11B values of representative sources of boron in ground water.
Tritium concentrations ranged from 7.0 to 10.3 tritium units in six samples from the surficial aquifer and were less than 0.8 tritium units in three samples from the basal sand aquifer. Water from wells in the surficial aquifer represents predominantly modern, post-1972 recharge and sources of boron and other constituents. Water from the basal sand aquifer is associated with pre-1952 recharge from sources not affected by local boron inputs.
Ground water from six wells (five domestic wells and one public-supply well) where the ground-water source was unknown had boron concentrations, boron isotope ratios, and tritium concentrations similar to water from the basal sand aquifer. Boron concentrations greater than the RAL were found in water from four of these six wells. The boron isotope and tritium data from these four wells indicate a natural source of boron in ground water; therefore, the RAL does not apply to boron concentrations in water from these wells. Water samples from two domestic wells where the ground-water source was unknown had boron concentrations less than the RAL and boron isotope ratios and tritium concentrations that were similar to domestic-wastewater-affected water from the surficial aquifer. The boron isotope ratio for a sample from one domestic well was similar to that of CCP-affected water from the surficial aquifer and detergent compositions; the boron concentration of that sample was less than the RAL. The classifications of differences among representative sources of boron in ground water and water samples from wells where the ground-water source was unknown generally agreed with distinctions based on strontium-87/strontium-86 ratios and concentrations of strontium, chloride, nitrate, and ammonia. This application of boron concentrations, boron isotope ratios, and tritium concentrations to classify differences in relation to potential sources of boron in ground water was able to distinguish between boron from natural sources and from human-affected sources that are subject to regulation.
ContentsAbstract Introduction Background Information Purpose and Scope Description of the Study Area Hydrogeologic Framework Methods of Data Collection and Analysis Well Selection Surficial Aquifer Wells Distant from Human-Affected Boron Sources Basal Sand Aquifer Wells Representing Natural Boron Sources Coal-Combustion-Product-Affected Wells in the Surficial Aquifer Domestic-Wastewater-Affected Wells in the Surficial Aquifer Water Samples from Wells with an Unknown Ground-Water Source Sampling Methods Laboratory Analyses of Water Samples Evaluation of Ground-Water and Boron Sources Quality-Assurance Results Boron and Boron Stable-Isotopes in Representative Ground-Water Sources Tritium in Representative Ground-Water Sources Evaluation of Ground-Water and Boron Sources for Wells with an Unknown Ground-Water Source Comparison with Selected Water-Chemistry Constituents Limitations of the Evaluation Method Summary and Conclusions Acknowledgments References Cited |
Figures
| 1–4. Maps showing: |
| 1. Study area near Beverly Shores and surrounding area, northwestern Indiana. |
| 2. Wells sampled in the study area near Beverly Shores, the Town of Pines, and the Indiana Dunes National Lakeshore, northwestern Indiana, 2004. |
| 3. Wells sampled and wetland areas in the study area near Beverly Shores, northwestern Indiana, 2004. |
| 4. Unconsolidated aquifer systems in the Lake Michigan region and the study area near Beverly Shores, northwestern Indiana. |
| 5. Diagrammatic hydrogeologic section showing aquifers and conceptual ground-water-flow directions in the western half of the Indiana Dunes National Lakeshore near Beverly Shores, Indiana. |
| 6. Map showing wells completed in the surficial aquifer and wells with an unknown ground-water source sampled near Beverly Shores, northwestern Indiana, 2004, in relation to the water-table altitude in the surficial aquifer, October 1980. |
| 7–11. Photographs showing: |
| 7. Wells 3S and 3B in relation to a nearby home along a dune ridge, facing southeast at Beverly Shores, Indiana. |
| 8. A flowing well developed in the basal sand aquifer at Beverly Shores, Indiana. |
| 9. Well 9A with the Yard 520 landfill in the background, facing southwest at the Town of Pines, Indiana. |
| 10. (A) View facing west of well 5W in the surficial aquifer at a National Park Service public facility, and (B) view facing southwest of homes that are upgradient of well 5W along the Lake Michigan shore at Beverly Shores, Indiana. |
| 11. An example of a suspected domestic-wastewater seep at Beverly Shores, Indiana. |
| 12–17. Graphs showing: |
| 12. The average monthly tritium concentration in precipitation in samples collected from Ottawa, Canada, 1953–2002, and from Chicago, Illinois, 1962–1979. |
| 13. Chemistry of water samples from wells in and near Beverly Shores, northwestern Indiana, 2004, in relation to boron isotope composition and boron concentrations for representative sources of boron in ground water. |
| 14. The average annual tritium concentration in precipitation, corrected for decay to July 2004, in samples collected from Ottawa, Canada, 1953–2002, compared with tritium concentrations in ground-water samples in the study area near Beverly Shores, northwestern Indiana, 2004. |
| 15. Boron concentrations (A) greater than 400 micrograms per liter and (B) less than 200 micrograms per liter in relation to boron isotope composition in water samples from representative sources of boron in ground water and from wells with an unknown ground-water source near Beverly Shores, northwestern Indiana, 2004. |
| 16. Tritium concentrations in water from wells with an unknown ground-water source that were (A) less than 1 tritium unit and (B) greater than 5 tritium units in relation to boron isotope compositions in water samples from representative sources of boron in ground water near Beverly Shores, northwestern Indiana, 2004. |
| 17. Strontium concentrations in relation to strontium-87/strontium-86 isotope ratios in ground-water samples for (A) representative ground-water sources near Beverly Shores, 2004, and slag-affected ground water in northwestern Indiana, 1997–99, and (B) water from wells with an unknown ground-water source near Beverly Shores, 2004. |
Tables
| 1. Comparison of hydrogeologic framework of the study area near Beverly Shores, Indiana with those of previous investigations. |
| 2. Selected characteristics of wells sampled for water chemistry near Beverly Shores and the Town of Pines, northwestern Indiana, 2004. |
| 3. Analytical methods for ground-water samples collected near Beverly Shores, Indiana, 2004. |
| 4. Determinations of field parameters for water samples collected from wells near Beverly Shores and the Town of Pines, northwestern Indiana, 2004. |
| 5. Water-chemistry determinations for samples collected from wells near Beverly Shores and the Town of Pines, northwestern Indiana, 2004. |
| 6. Water-chemistry determinations for samples and sequential duplicates collected from wells near Beverly Shores and the Town of Pines, northwestern Indiana, and for deionized water and an equipment blank, 2004. |
| 7. Comparison of boron determinations from analyses of acidified and unacidified samples from wells near Beverly Shores and the Town of Pines, northwestern Indiana, November 2004. |
| 8. Boron and boron stable-isotope analyses of a borax detergent additive and a detergent with perborate bleach, 2004. |
| 9. Ranges of boron isotope ratios in samples of representative ground-water sources of boron collected in the study area near Beverly Shores, northwestern Indiana, 2004 and of detergent additive and detergent samples, 2004, as compared to selected published data. |
| 10. Comparison of chemistry of domestic-wastewater-affected water samples to those from a hypothetical mixture of representative compositions of water from the surficial aquifer and water affected by coal-combustion products. |
| 11. Classifications of similarity to representative sources of boron in ground water and to ground-water source, based on boron isotope compositions and boron and tritium concentrations. |
| 12. Water-chemistry determinations for slag-affected samples collected from wells in northwestern Indiana, 1997 and 1999. |
Availability
This document is available in Portable Document Format (PDF)
To view and print report you will need to use Adobe Acrobat Reader (available as freeware)
Users with visual disabilities can visit Online conversion tools for Adobe PDF documents web page
Whole report (11.8 MB) - 46 pages (8.5' by 11' paper)
Suggested Citation:
Buszka, P.M., Fitzpatrick, J., Watson, L.R., and Kay, R.T., 2007, Evaluation of ground-water and boron sources by use of boron stable-isotope ratios, tritium, and selected water-chemistry constituents near Beverly Shores, northwestern Indiana, 2004: U.S. Geological Survey Scientific Investigations Report 2007–5166, 46 p.
For more information about USGS activities in Indiana, visit the USGS Indiana Water Science Center home page.
| U.S. Department of the Interior, U.S. Geological Survey Persistent URL: Page Contact Information: Contact USGS Last modified: Thursday, December 01 2016, 07:52:14 PM |
